The polygenic liquid biopsy test
Com.Pl.it DX® Liquid Breast
The Com.pl.i.t DX Liquid Breast assay is tailored for postmenopausal women who have recurrent or metastatic breast cancer with (ER+) and HER2-. This assay is a strong indicator that determines if these patients should receive particular targeted therapies.
The Com.pl.i.t DX Liquid Breast Test is suitable for:
- Patients with non-operable tumors and patients with limited or inadequate tissue biopsy material.
- Patients with multiple metastases.
- Patients under treatment or after completion of treatment. In this case it gives insight in the eventual arise of new targetable mutations or resistance mutations to the treatment used.
To perform the Com.Pl.it DX® Liquid Breast Multi-Gene Biopsy Test, we only need a small amount of blood from the patient, for which a normal routine draw is made and placed in a special Streck™ vial provided by Genekor.
The Com.pl.i.t DX Liquid Breast Test:
- Determines the molecular profile of the tumor such as gene mutations and Copy Number Variations for inoperable tumors.
- Designates the on-label drug that targets the mutated gene(s) or the pathway that the gene(s) are involved.
- Identifies the mutations associated with resistance to targeted treatment.
- Recommends off-label therapies and/or indicates therapies that are currently in clinical trials.
In more detail, the test is highly recommended for targeted treatment decisions for:
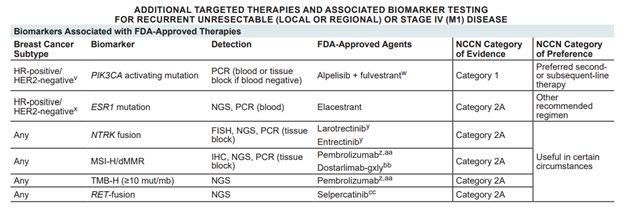
- Postmenopausal women with advanced or metastatic ER+/HER2- breast cancer after relapse on prior therapy, in order to decide on treatment with Elacestrant, based on ESR1 gene mutations.
- Postmenopausal women with advanced or metastatic ER+/HER2- breast cancer after relapse on prior therapy, in order to decide on treatment with Alpelisib, based on PIK3CA gene mutations.
Postmenopausal women with advanced or metastatic breast cancer relapse on prior therapy, in order to decide on off-label or clinical trial treatments.
Table of Genes Analyzed
10 gene alterations
| AKT1 | EGFR | ERBB2 (HER2) | ERBB3 | ESR1 |
| FBXW7 | KRAS | PIK3CA | SF3B1 | TP53 |
Copy Number Variations- CNVs
| CCND1 | ERBB2 (HER2) | FGFR1 |
In the gene panel above, we detect mutations in the ESR1 gene (incidence 48% as a mechanism of resistance to hormone therapy, based on the Emerald study).
We also detect PIK3CA mutations with the approved treatment Alpelisib, as well as other genes for which there are experimental or off-label treatments.
Συχνές ερωτήσεις
Com.Pl.it DX® Υγρή βιοψία Μαστού
BIOMARKERS ASSOCIATED WITH FDA-APPROVED THERAPIES